Products & Services
ION EXCHANGE
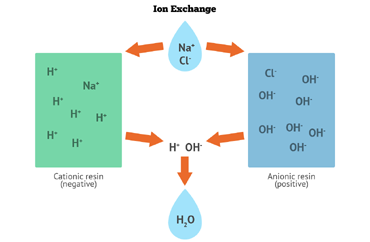
ION EXCHANGE SYSTEM
As the name implies Ion exchange processes are reversible chemical reactions where ions of same charge are exchanged between two medium. It is primarily used in water treatment systems for removal of dissolved ions and replacing them with similarly charged ions. Charged synthetic resins or naturally occurring materials(zeolites) act as the surface for the exchange process to occur.
In a cation exchange process, positively charged ions in the water are exchanged with positively charged ions on the resin surface - typically sodium. Whereas, in anion exchange, negatively charged ions in the water are exchanged with negatively charged ions on the resin surface - typically chloride. Contaminants such as nitrate, fluoride, sulfate, and arsenic, as well as others, can all be removed by anion exchange. Water softening is the most widely used cation exchange process.
Demineralization
Process of removal of all minerals from the water by use of ion-exchange mechanism.
Dealkalisation
Process of removal of all alkalinity causing ions from the water by use of ion-exchange mechanism.
Water Softener
Works on the principle of removing calcium, magnesium and other metal cations that causes hardness in the water to render the outlet water soft.
 AQUATREAT ENGINEERING PVT. LTD.
AQUATREAT ENGINEERING PVT. LTD.